
YEAST AND BALLOONS |
|
Objective of the experimentWe want to observe which ambient favours the fermentation of yeast InstructionsPut in three test tubes the following substances (use the same amount of each substance): test tube # 1 : water + yeast Quickly fix a balloon
on the mouth of every test tube. |
 |
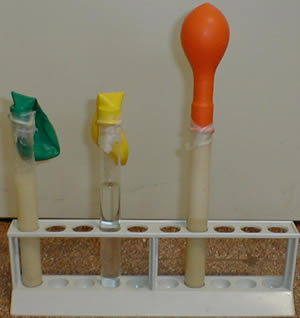 |
Observationstest tube # 1 : nothing happens ConclusionsThe balloon swells
because of the formation of a gas produced by the yeast through
the fermentation.
In order to ferment the yeast needs sugar. |
BICARBONATE, VINEGAR AND BALLOONSObjective of the experimentWe want to observe what happens stirring sodium bicarbonate and vinegar. What to make
ObservationsFoam is formed in the beacker and the balloon swells. ConclusionsBy stirring the two substances a gas is developed like in the
case of the yeast with the sugar, but in this case the balloon
has swollen
more because the gas is much more abundant. In some recipes,
bicarbonate mixed with an acid is used instead of yeast. |
||
 |
 |
 |
| School site | Aristotle | Redi | Spallanzani | Metamorphosis | Microorganisms | "The tiny animals" | |